Parenting(Age 5 to 8) | Parenting(Age 9 to 12) | Parenting(Age 13 to 16) | Dec 02, 2016
periodic table
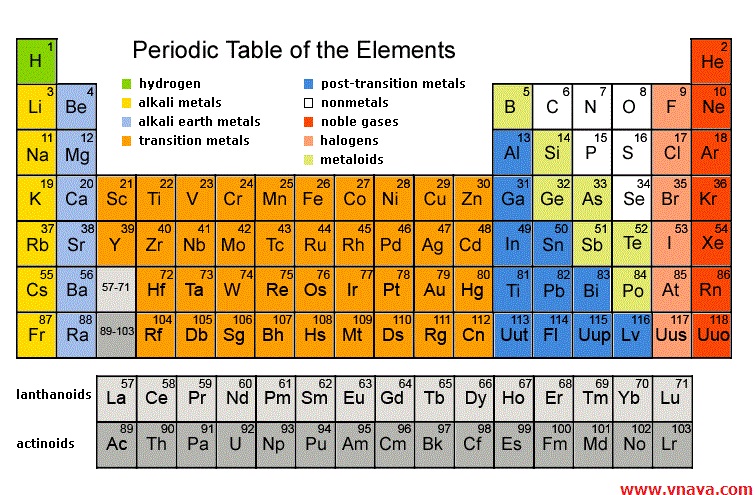
INTRODUCTION OF PERIODITIC TABLE
We know that right now about 115 elements are found in our earth crust. And they all are arranged in a tabular arrangement and that is called Periodic table. “Mendeleev” is called the Father of Modern Periodic table.
In Periodic table all elements are arranged in their order of increasing atomic number and their increasing atomic structure.
Example: We can take example of hydrogen and helium to prove it as:
| HYDRODEN | HELIUM |
1) The Atomic Number of Hydrogen is = 12) The atomic structure of Hydrogen is : 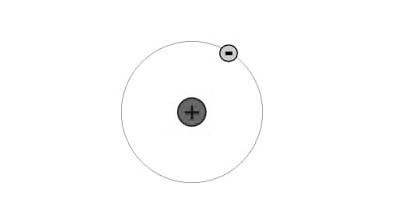 H is having only One electron. And its Atomic structure is smaller than He. So H is placed at 1 position. H is having only One electron. And its Atomic structure is smaller than He. So H is placed at 1 position. | 1) The Atomic number of Helium is = 22) The Atomic Structure of Helium is : 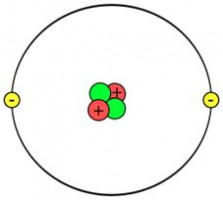 He is having atomic number =2 .And its atomic structure is bigger than H. So it is placed in 2 positions in periodic table. He is having atomic number =2 .And its atomic structure is bigger than H. So it is placed in 2 positions in periodic table. |
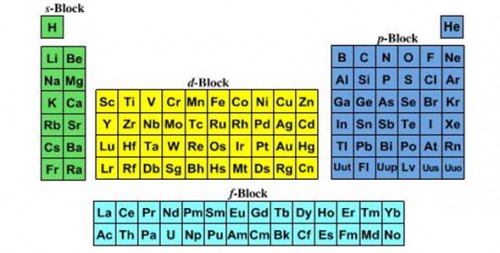 ARRANGMENT:
ARRANGMENT:Elements are arranged in two ways in periodic table these are:
Row------The rows of the periodic table is called Period.The periods are the Horizontal lines Which varies from :
L.H.S. =R.H.S.
And there are 7 Periodsin our periodic table.
Column ----Column in the periodic table is called Group. The groups are vertical lines and they vary from:
Top to bottom
There are 18 groupsin our periodic table.
CLASSIFICATION OF PERIODIC TABLE: Periodic table is mainly divided into four blocks these are:
s-Block
p-Block
d-Block
f-block
EXPLANATION: S-block element: The elements in which the last electron is entering into s- sub shell are called s block elements. The s-block elements are present on the left side of periodic table. All elements in Green color are s-block element.
Example:
H=1s1 Li=1s22s1
K= (Ar) 18 4s1
Ca= (Ar)18 4s2
The electronic configuration of s-block always be “ns2”. Because s block element can holds maximum of 2 electrons as:
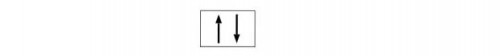 But both having opposite spin.
But both having opposite spin.p- Block elements: The elements in which the last electron is enter into p-orbit called p-block elements. The p-block elements are present on the Right side of periodic table. He is not considered as p-block element because p-block having more than 3e in their outermost shell. They can carry maximum of 6 electrons.
Example: B= (He)2 2s2 2p1 C= (He)2 2s2 2p2 S= (Ne)10 3s2 3p4 Etc. So their electronic configuration is ns2 np6.
So that means p block can hold maximum of 6 electrons.
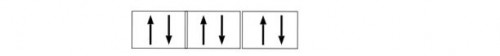
d- block: The elements in which the last electrons enter in d sub shell they are known as d-block elements. These are present in the middle of periodic table and these are vary from:
Sc to Zn
Y to Cd
Lu to Hg
Lr to Cn
The d-block elements can carry maximum of 10 electrons.
Examples:
Fe = (Ar) 183d64s2
Co= (Ar) 183d74s2
Zn= (Ar) 183d104s2
So the electronic configuration of d-block elements is
ns2, (n-1)1-10
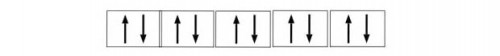 So d-block can hold maximum of 10 electrons. The elements in d-block are also called Transition Elements.
So d-block can hold maximum of 10 electrons. The elements in d-block are also called Transition Elements.f-block: The elements inwhich last electron will enter into f-block is called f-block elements. These elements are present at the bottom of the periodic table in two rows.
They are also called Inner transition elements.
In this the last electron is enters in f-block.
Example:
La = (Xe) 54 5d1 6s2
Yb = (Xe) 54 4f14 6s2
The outermost electronic configuration of f-block elements is
(n-2)f1-14(n-1)d0-10ns2
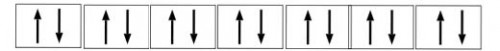 So the f-block can occupy maximum of 14 electctrons.
So the f-block can occupy maximum of 14 electctrons. For Online Chemistry Tutoring and Tutors click here.





.jpg)









Post a Comment: